How DMI-65® Works as Filtration Media: The purpose of this paper is to provide users of DMI-65 catalytic water filtration media with qualitative information about how the material works, its capabilities and limitations and enable them to apply the material to water treatment processes in the appropriate manner and with confidence. The paper avoids the detailed complexity of solid surface electrochemical layers and colloidal science, quantitative physical and chemical processes and reactions. For readers having already significant knowledge in this area the paper brings more understanding of what DMI-65 is and its intended use. Newcomers to this area are provided with the bases, and perhaps motivation, for directing their more in depth studies as they might wish.
History
How DMI-65® Works compared to other products: In the early days of water treating, naturally occurring zeolites (such as glauconite greensand) were used to soften and remove the iron and manganese from boiler make-up and process waters. As the demand for higher quality water increased (due in part to higher pressure class boilers) the water treating industry largely moved away from these products for softening to the newly developed synthetic ion exchange resins.
However, in the case of iron and manganese removal this move was much slower and the result was that the use of glauconite greensand (greensand) filtration media continues until the present time. Greensand was and is often used as a pretreatment step prior to ion exchange processes since the iron in a feed water can and does foul the cation resin. Other processes include aeration and oxidation-filtration with standard media filters or proprietary types of media and/or filters
While there have been other iron/manganese removal products and processes developed since greensand was introduced the use of greensand continued even though there were several issues that made it a less than ideal media. It required periodic regeneration with potassium permanganate, could not be used in lower pH waters (<6.2), had a relatively low operating temperature (80oF), and tended to soften through time resulting in pressure drop issues at higher flow rates. Additionally, the supply could occasionally become restricted due to environmental concerns with the processing facilities along the Eastern coast of the United States.
Because of these issues in the 1970s water treating companies and end-users began to express an interest in “something else” to replace the greensand. In response to their requests, scientists and researchers in Japan began to look for ways to infuse oxidizing agents to different matrix materials. It was felt that a commercially produced product could be made more powerful, have better physical properties and be more subject to improvements and/or modifications than any naturally occurring media.
Decades of further research and development of the Japanese Infusion Technology have resulted in the uniquely Australian made product, DMI-65 a granular catalytic media used to boost the advanced reduction/oxidation (redox) processes in water. The media is part of a broad category of products deriving their physical and chemical action from the interaction of their metal oxide surface with the water molecules and ions in solution. This product is revolutionary due to proprietary infusion technology that penetrates the micro pours substrate of the matrix material, allowing for a greater catalytic surface area and of a tight particle size distribution. DMI-65 has low level of fines, a tolerance to wider pH range and chemically infused catalytic surface that won’t be consumed or diminished under normal operating conditions. Last 5 – 10 years of continuous use.
Background information
How DMI-65® Filtration Media Works: It is an extremely powerful catalytic water filtration media that is designed for the removal of iron and manganese in aqueous solutions (water) without the need for potassium permanganate or chemical regeneration. The unique microporous structure of DMI-65 efficiently removes dissolve iron to the almost undetectable levels as low as 0.001 ppm and manganese to 0.001 ppm. DMI-65 acts as an oxidation catalyst with immediate oxidation and filtration of the insoluble precipitates derived from this oxidation reaction. DMI-65 can also remove Arsenic, Aluminium and other heavy metals and Hydrogen Sulfide under certain conditions.
The material is part of the broad category of products deriving their physical and chemical action from the interaction of their metal oxide surface with the water molecules and ions in solution.
Solid surface interaction with water distinguishes between adsorption as the weak van der Waal forces that hold a hydrophobic molecule in a rigid core media such as activated carbon and absorption as the weak van der Waal forces that hold a hydrophobic molecule in a swellable matrix (such as benzene) in a polymer of T-butyl styrene or absorption by liquid-liquid extraction. Ion exchange resins utilize absorption processes while interaction of DMI-65 with water molecules and ions in solution is initiated through adsorption.
Non catalytic type adsorbent materials retain target ions from water until either sites available for adsorption reach a maximum density and saturation or concentration of target ions in the treated water attain maximum acceptable concentration. At this point, the adsorbent material has to be regenerated to remove or replace the contaminant ions, or the used material is replaced with new material that is loaded in the treatment container. When the process works by swapping one type of ion for target ions from water the process is called ion exchange. This category of adsorbent and some partly absorbent materials remove the target ions from water. The larger the surface per volume of material the larger the amount of contaminant target ions which could be retained from the water.
Purely catalytic materials adsorb the reactant ions from solution bringing them in the proximity of chemical bonding. Then the reaction product moves away from the surface of catalyst. Strictly speaking catalysts facilitate chemical reactions; they do not implicitly remove anything. If the reaction product is a solid precipitate, often the product is retained in the catalytic bed, hence removed by filtration.
Many materials act in a mixed mode; with both ion exchange and catalytic action taking place. For those materials used primarily for their catalytic action, ion exchange resulting in dissolution of the catalytic layer leads to the need for periodic regeneration or reactivation to correct the matrix of ions at their active surface.
How DMI-65® Works – Advanced Oxidation Catalytic Media
DMI-65 is a granular material of dark brown to black colour. This colour is produced by the manganese oxide in the outer layers of the granules. DMI-65 is a catalytic media in the true meaning of the word and facilitates oxidation – precipitation – filtration and does not get consumed in the reactions. Strictly speaking, the media facilitates chemical reactions and does not explicitly remove anything. Once oxidized, the depth filtration aspect of the media removes the solids that are then periodically backwashed out of the filter vessels.
The filtration media does not need regeneration or reactivation and does not display a decaying capacity to do its catalytic work. Over 5 to 10 years period, through many backwashing operations of the bed to remove retained solids, the media is degraded by contact between particles and mechanical abrasion. Then the material has to be replaced.
HOW DMI-65® WORKS: BASIC OPERATION:
The processes that take place in a bed of DMI-65 involve reduction/oxidation (redox). Redox reactions involve a transfer of electrons between species. Reduction is the gain of electrons or a decrease in the oxidation state of a molecule, atom or ion. Oxidation is the loss of electrons or an increase in the oxidation state of a molecule, atom or ion. Redox reactions occur simultaneously whereby there cannot be a reduction reaction without an oxidation reaction. The media “helps” chemical reactions to occur by interacting with the reaction without being permanently altered. An in depth discussion about redox chemistry is outside the scope of this paper, it will only deal with how the redox process applies in the removal of iron and manganese using DMI-65. The individual redox equations will be covered in the following iron and manganese removal sections.
In order to begin the process of oxidation of the ions in solution and to ensure that the oxidative layer is not compromised the media is designed to operate in the presence of chlorine or other oxidant. In this process the oxidant removes electrons and is consumed in the process. The operator needs to ensure that there is a 0.1 – 0.3 ppm free chlorine residual in the effluent water.
Chlorine, fed as sodium hypochlorite (NaOCl) or bleach (12.5% NaOCl), is the preferred oxidant since it is relatively inexpensive, readily available around the world and it is effective. Other oxidants such as hydrogen peroxide (H2O2), chlorine oxide (ClO2) or ozone can also be used so long as a residual can be measured and maintained.
Another function of the chlorine is that it keeps the media free from bacterial or slime growth. The manganese oxide catalytic surface has to remain clean so that the ions in the water can come in contact with it. At the same time, the chlorine is a source of oxygen more reactive than molecular oxygen. The following chart indicates safe levels for other water constituents that could interfere with the surface interaction.
Unlike ion exchange resins where higher regenerant dosages will increase the ion exchange capacity, chlorine residuals or concentrations higher than required to oxidize the Fe and Mn do not increase the oxidative properties of the media. Additionally, since the media is often used to pretreat waters prior to an Reverse Osmosis (RO) system a higher free chlorine residual would require more extensive post treatment to reduce the residual to protect the membranes from chlorine attack.
The DMI-65 must be activated prior to being placed into service for the first time. This activation requires a higher dosage of chlorine than used during normal operation but only has to be performed once during the initial start up. The dosage rate is 10 fluid ounces of 12.5% chlorine per cubic foot (ft3) of the media. The activation only requires a soak of several hours but an overnight soak is preferred.
Once activated, the vessel(s) must be backwashed to remove the excess NaOCl and any fines. Since manganese oxide is one of the constituents used in the manufacture of the media an extended rinse is required at start up to remove any trace free manganese oxide residual left over from the manufacturing process. Once the Mn level in the backwash water reaches values of 0.05 to 0.15 ppm and the free chlorine residual is set the filter is ready to be placed into service.
Media replacement due to the decreased physical filtration properties of the Media due to physical abrasion will occur before complete degradation of the catalytic layer takes place. Under normal operating conditions media life is estimated at 5 – 10 years.
How DMI-65® Works for removing Iron (Fe) precipitation and removal using DMI 65
Iron (Fe) is the fourth most common element found in the earth’s crust and exists in a wide range of oxidation states from -2 to +6 although the most common states are ferrous (+2) and ferric (+3).
Ferrous salts are readily soluble. Before the ferrous iron, a dissolved solid commonly found as ferrous bicarbonate, can be removed by filtration it must be oxidized, become ferric hydroxide and in neutral pH waters precipitate out in the media bed. The catalytic surface of DMI-65 contains manganese oxide or exposes manganese and oxygen sites for adsorption of [Fe] ions that are in the water. The reaction of ferrous bicarbonate and NaOCl is almost instantaneous and the ferrous bicarbonate oxidizes (gives up an OH–) to become the insoluble ferric hydroxide which is then removed through filtration in the catalytic surface of the media. The following redox reaction equation explains the process.
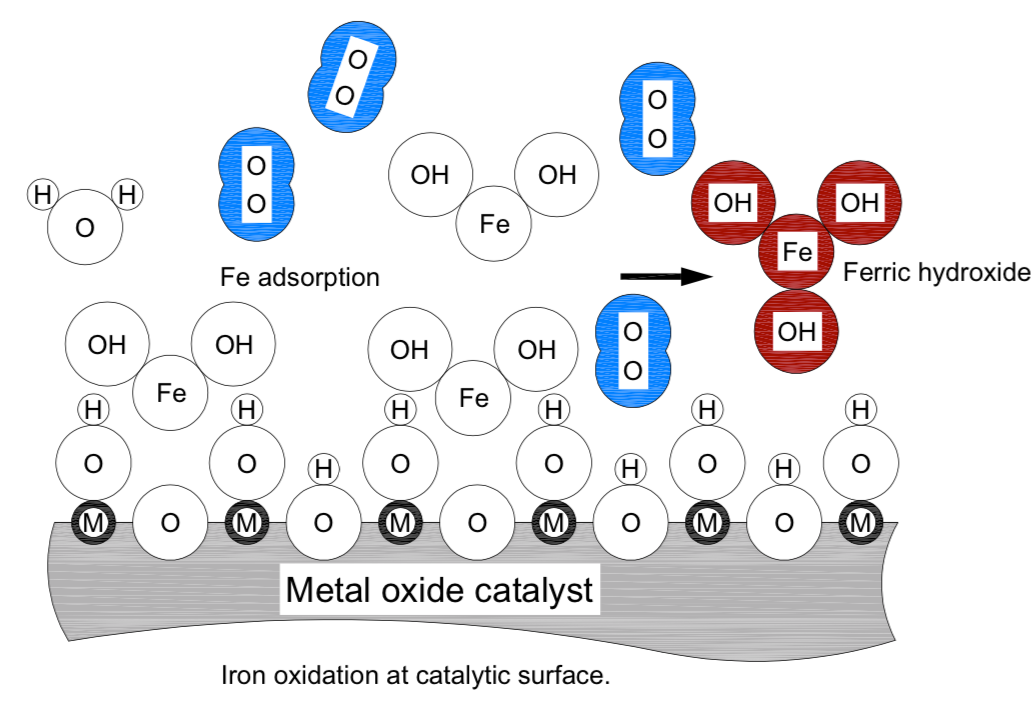
2Fe(HCO3)2 + NaOCl + H2O + 2Fe(OH)2 + 2CO2 + NaCl
In the above figure the catalytic surface is presented in a simplified smooth form. Letter “M” was used to represent a generic metal ion in the lattice of this surface. Letter “O”, in the centre of circles, represents an oxygen atom. Various ion size and oxygen molecule (blue) are represented at true relative scale. Except the oxygen molecule, bonded irons are shown as tangent circles. The interpretation for letters and ions in the figure “Ion oxidation at catalytic surface” is:
M: generic metal ion in the catalytic surface lattice (Mn+); n = 1, 2…
O: oxygen atom or ion (O-)
Fe: iron atom or ion (Fe2+, Fe3+)
H: hydrogen atom or ion (H+)
OH: hydroxide, or hydroxyl anion (OH-)
H2O, water molecule shown as tangent circles
Fe (OH)2, ferrous hydroxide is shown as tangent circles
Fe (OH)3, ferric hydroxide, shown as tangent circles, brown colour
O2, oxygen molecule, atoms shown at covalent bonding distance, blue colour
Dissolved ferrous bicarbonate is attracted with the Fe end towards the lattice oxygen of the catalytic material. This brings the Fe in the proximity of covalent bonding with the hydroxide ion of a neighbouring site and the ferrous bicarbonate changes into insoluble ferric hydroxide which precipitates in crystalline form aggregates of size from 3 nanometre and larger. The aggregates coagulate in larger flocks and are retained in the catalytic bed.
As the ferrous bicarbonate is converted into ferric hydroxide, its concentration at the catalytic surface decreases. In the bulk of the water, away from the catalytic surface, the concentration of ferrous bicarbonate is higher resulting in it diffusing towards the lower concentration according to diffusion law. Diffusion flux is linearly dependent with concentration gradient over distance.
Dissolved oxygen contributes to production of hydroxide ions through direct oxidation of hydrogen in combination with Fe splitting the water molecule and by reacting with the hydrogen at the catalytic surface
It is important to note that although a source of oxygen is needed oxidation and precipitation of Fe is driven by the hydroxide ion. Even under relatively acidic conditions hydroxide ions (a very strong anion) are easier available for binding to Fe than oxygen. Thus, Fe is not very difficult to oxidise and precipitate around neutral pH condition. In addition, concentration of hydroxyl ions increases with pH value exponentially and so does the rate of oxidation and precipitation of Fe.
Chlorine (usually in the form of NaOCl) is a source of oxygen more reactive than molecular oxygen. The ideal residual to be maintained downstream of the catalytic filter is 0.2 mg/l (0.1 to 0.3) free chlorine. A higher residual of free chlorine and therefore higher sodium hypochlorite level in the catalytic filter does not always help. It could have an adverse effect due to venting of chlorine and an increase of competing sodium ions, Na+. The catalytic surface has to be clean so that ions in water could come in contact with it so the chlorine injected also prevents bacteria growth and blinding of the catalytic surface with slime.
How DMI-65® Works for removing Manganese, Mn precipitation
DMI-65 is a catalytic material specifically tailored to the oxidation and removal of manganese. The catalytic surface contains manganese oxide for brining into proximity of covalent bonding manganese and oxygen atoms from water. However, oxidation and removal of manganese (Mn) is vastly different from that of Fe. A major difference is caused by the solubility of manganese oxyhydroxide, MnO(OH)2.
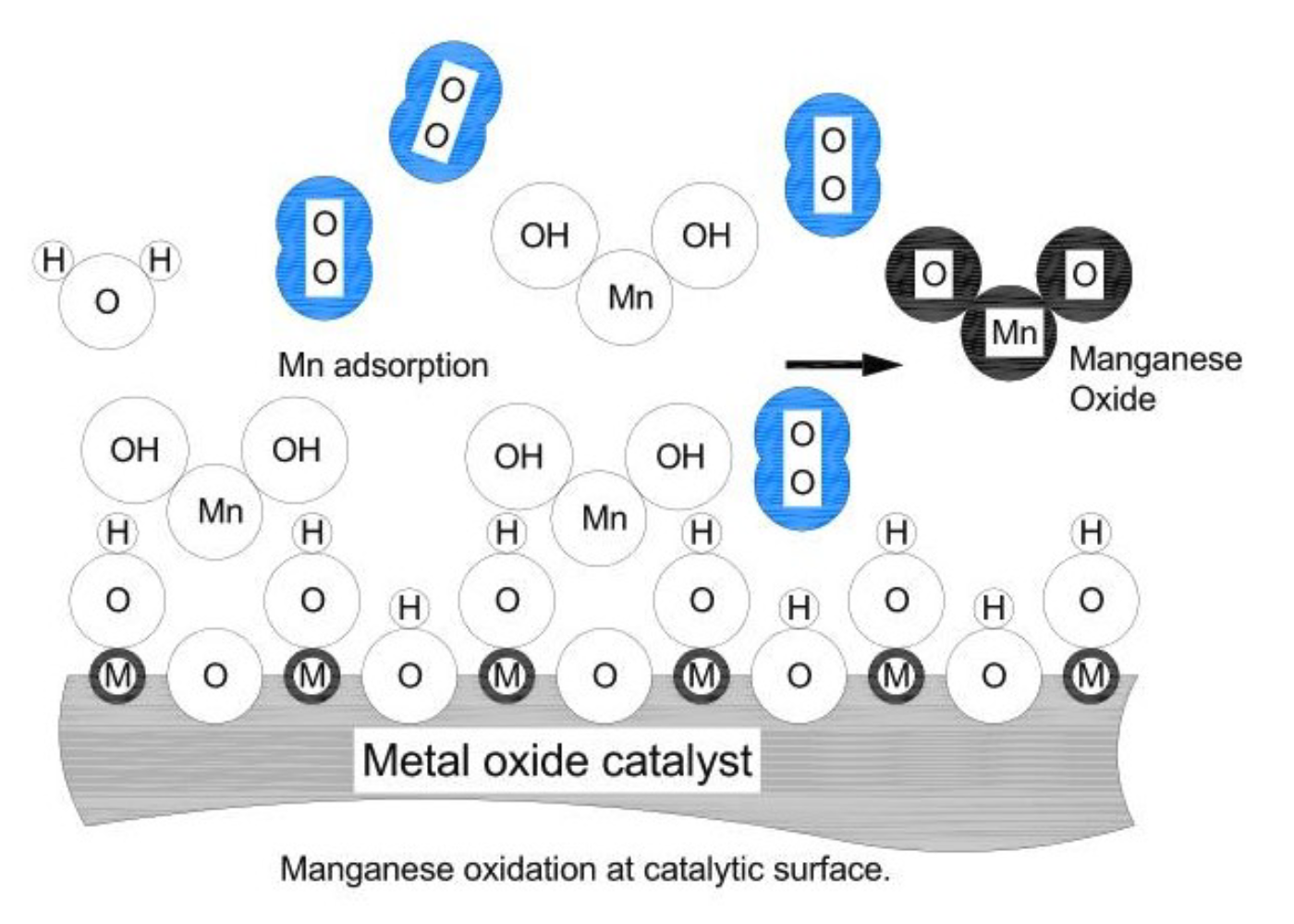
Mn(HCO3)2 + NaOCl + MnO(OH)2 + NaCl + 2CO2
Manganese does not precipitate as oxyhydroxide but as oxide, MnO2 and higher valency oxides. Presence and concentration of hydroxide anions does not help much in the precipitation and removal of manganese. Manganese hydroxide will be attracted with the manganese end to the oxygen in catalytic lattice surface. An Oxygen molecule has to be available in the proximity for facilitating oxidation through the oxygen from lattice and swapping to the lattice with molecular oxygen. Conditions for this to happen are statistically less probable and reaction is of much slower rate than the oxidation of Fe via hydroxide.
While increases in pH to alkaline levels facilitates oxidation and removal of manganese, under these conditions the oxidised manganese could also dissolve back into the water. Consequently, regardless of the target contaminant to be removed, anoxic conditions have always to be avoided to protect the catalytic layer against leaching into water. When oxidising manganese the recommended pH is close to 8.
Manganese oxide has good autocatalytic effect. When backwashing it is better to stop the process before the water becomes very clear. Manganese oxide residue in the filter bed will enhance manganese oxidation.
How DMI-65® Works : Key operating conditions
Treatment processes have to be conducted in such manner so that the catalytic surface of the material is kept clean and available to ion from water to contact.
Water with a large amount of suspended solids has to be clarified before passing it through the catalytic filter with DMI-65. Acceptable levels of suspended solids depends on their nature. A larger amount of mineral suspended solids than organic suspended solids could be handled.
Bacteria could grow and deposit slime on DMI-65. Thus disinfectant and oxidation conditions have to be maintained.
Water containing clays and large organic molecules may result in deposition of such material on the surface of DMI 65 and blinding of the catalytic surface. Treatment for removal of such contaminant before the catalytic filter is needed.
Polymer flocculent could also stick to the DMI-65 and blind catalytic surface.
Hard, unstable groundwater could cause scale deposition in the catalytic filter and blind the material in a solid mono bloc. In such case the DMI-65 material in the bed is lost and would have to be replaced. Treatment for stabilizing the water to prevent scale formation in the catalytic filter has to be carried out.
Both low acidic pH and anoxic conditions could cause dissolution of manganese from catalytic layer of DMI-65 and loss of its capacity. Excessively high pH means excessive concentration of hydroxyl ions (corrosive to metals) and could also cause dissolution of manganese from the catalytic layer.
Do not use demineralized water, distilled water or water known to be strongly corrosive to metals for initial soaking and activation of DMI-65.











